Research
|
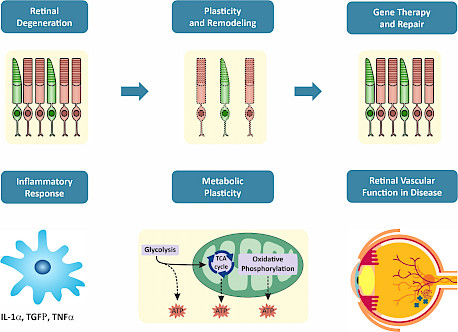
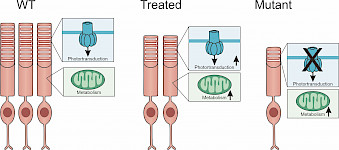
Research Topics Our research aims to 1) identify the molecular mechanisms driving photoreceptor degeneration and retinal remodeling, with the potential to be tackled therapeutically and 2) identify targets and develop preclinical models to test candidate gene therapies for their ability to prevent photoreceptor degeneration and vision loss. We use a variety of preclinical models and combine molecular, biochemical, and electrophysiological approaches with fluorescence imaging and immunochemical analysis. Inflammation in Degenerative Retina |
||
|
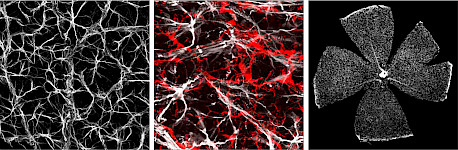
The disease pathology in RP is mostly driven by mutations in the light-sensitive rod photoreceptors, which trigger their death and, secondarily, the death of cone photoreceptors. While the mechanisms driving the “bystander” cone degeneration in RP have not been defined, inflammation has long been implicated. The inflammatory response is characterized by the activation of microglia and Müller glial cells expressing a variety of receptors that allow them to detect environmental changes and initiate inflammatory pathways. We hypothesize that increased inflammation in degenerating retinas increases the speed of cone photoreceptor cell death, and reducing inflammation by targeting multiple pathways can slow retinal disease progression.
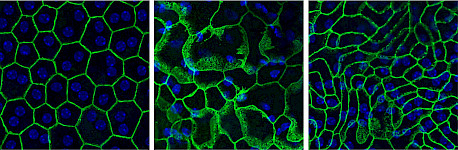
RPE Plasticity in Retinitis Pigmentosa Photoreceptor degeneration leads to cellular and molecular remodeling of retinal pigment epithelium (RPE). The RPE is a monolayer of hexagonal pigmented cells interconnected via tight junctions and located between the photoreceptor layer and the choroidal blood vessels. In healthy retina, RPE cells support the health and function of photoreceptor cells, including phagocytosis, protection and metabolic support. Understanding the molecular and structural changes in RPE during retinal degeneration is of crucial importance to enable new therapeutic advances.
Metabolic changes
Photoreceptors require high levels of glucose to sustain the constant outer segment morphogenesis and to preserve membrane excitability. It has been suggested that photoreceptors rely on aerobic glycolysis (aka the Warburg effect) - an unusual metabolic pathway that supports the unrestrained cell proliferation in cancer cells. In aerobic glycolysis, most glucose is converted to lactate rather than catabolizing it completely to carbon dioxide via oxidative phosphorylation. In photoreceptors, perturbations targeting key regulators in the glycolytic pathway impact photoreceptor function and survival. |
|
|